
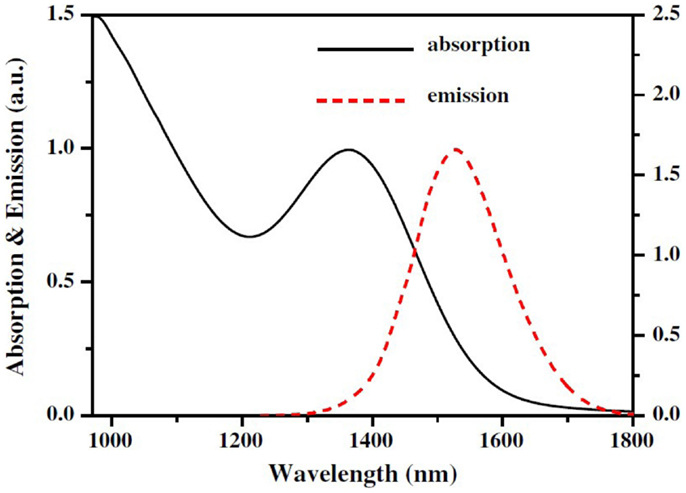
Ĭ-dots are easily dispersed in protic and aprotic solvents due to carboxyl, hydroxyl, and carbonyl groups. Also C-dots have gained widespread attention in recent years, especially in chemical censoring, biosensing, bioimaging, drug delivery, solar cells, light-emitting diode (LED), and electrocatalysis. fabricated chemiluminescence C-dots and used it to develop new class of CL nanosensors for the imaging Reactive oxygen species. Owing to their physicochemical properties, C-dots can take part in the chemiluminescence reaction as oxidants, emitting species, energy acceptors of chemical reaction energy or even as catalyst involving in different chemiluminescence systems. The synthesis approaches of C-dots can be classified into two categories: top-down and bottom-up methods including hydrothermal, electrochemical oxidation, acidic oxidation, microwave, and laser ablation. In recent years, different materials and synthesis methods have been used to obtain C-dots. C-dots was first discovered by electrophoretic purification of single-walled carbon nanotubes in 2004. Also, introduction of full-color fluorescent C-dots is another advantage that can expand their application spectrum. C-dots are heavily fluorescent, non-blinking, water soluble, chemically stable and can be easily synthesized at low cost. These shortcomings can be addressed by carbon dots (C-dots) which are spherical carbon-based nanoparticles with a size of less than 10 nm. Finally, the quantum yield of C-dots in the eclectic aprotic solvents was communicated and discussed.Ĭarbon-based nanomaterials such as carbon nanotubes, fullerene and graphene have poor solubility in water and lack strong fluorescence in the visible area, limiting their applications. The results showed that the dipole moment of the excited state is more than the ground state, indicating a high density and redistribution of electrons in the excited state. The values found for μ g = 1.077 D, and μ e = 3.157 D, as well as the change in the dipole moments. The difference between the dipole moment of the excited state and the ground state was shown using an extended form of Lippert equations by Kawski and co-workers. The change in the value of dipole moment was estimated by using the Stokes shifts. In this research, we report on the effect of aprotic solvents on absorption and fluorescence spectra and dipole moments of C-dots dispersed in a range of many aprotic solvents with various polarity and dielectric constant at room temperature. ResultsĬarbon dots with intense photoluminescence properties have been synthesized by a one-step hydrothermal method from a carbon bio-source. For reaching our aims, C-dots were synthesized and dissolved in the different solvents. Today, many studies are performed to exploit the photoluminescence (PL) property of carbon dots, and our focus in this study is to estimate the dipole moment of carbon dots. Carbon dots (C-dots) are photoluminescent nanoparticles with less than 10 nm in size.


 0 kommentar(er)
0 kommentar(er)
